My group is currently implementing 4 experimental research projects financed by EU and Romanian national research funds. Apart from this, we developed an Elisa assay for the detection of SARS-CoV-2 IgG antibodies, which is now a CE IVD marked product of our startup, Proel Biotech.
1. Implementing a multiplex methodology for rapid testing of hepatic viruses (MULTIHEP)
Project permalink: click here
This project is carried out as a joint partnership of Pro-Vitam Ltd (project leader) with the Institute of Biochemistry of the Romanian Academy of Sciences (IBAR, project partner), and financed by CCCDI-UEFISCDI (project number PN-III-P2-2.1-PTE-2019-0226). Project activities are co-financed by Pro-Vitam Ltd. The project runs from May 2020 to May 2022.
Total project budget: grant of 1,189,000 RON (CCCDI-UEFISCDI) and co-financing of 158,960 RON (Pro-Vitam Ltd.). The project is financed through the National Research and Development Program III scheme entitled “Program 2 – Increasing the competitivity of the Romanian economy through research, development and innovation”.
Project manager: Dr Szilard Fejer
Director of research of the partner institution: Dr Costin-Ioan Popescu
Project details
Hepatic viruses represent a major global health problem worldwide with more than 325 million people being chronically infected. Hepatitis C Virus (HCV), Hepatitis B Virus (HBV) and Hepatitis Delta Virus (HDV) induce chronic infections and liver pathology ranging from liver steatosis to cirrhosis. The standard of care for HCV has been recently upgraded with direct acting antivirals being approved for clinical use. The present treatment for HBV is able to control the viral infection, but it does not achieve the clearance of the viral genome. HBV/HDV coinfection determines the most serious form of hepatitis and there is no specific antiviral treatment. In Romania, there is a high prevalence of HBV/HDV coinfected people. Thus, the diagnosis of patients infected with hepatic viruses and their early access to treatment represents a public health priority. Rapid detection tests (RDTs) represent an efficient method to achieve that goal. The present project aims to develop a multiplex RDT for detection of anti-HCV, anti-HDV and HBsAg in patient sera. Our test will use the HDsAg as a capturing probe for anti-HDV antibodies. HCV and HDV antigens will be produced in a recombinant prokaryotic system. Using these antigens, a multiplexed RDT will be assembled and tested in the research laboratory and medical testing laboratory as a prototype.
Group members
Pro-Vitam (project coordinator):
- Dr Szilard FEJER
- Dr Zsuzsanna JENEI
- Dr Kinga RAKOSI
- Monika KORODI (PhD student)
IBAR (project partner):
- Dr Emil NEAGA
- Dr Paula Ecaterina FLORIAN
- Lia CUCOS (PhD student)
- George Nicolae Daniel ION (PhD student)
- Alina Veronica GHIONESCU (PhD student)
Summary of research
The main activities undertaken in phase 1 of the project are the following:
- expression and purification of a Hepatitis Delta virus antigens (IBAR)
- development of an Elisa protocol for detection of HCV and HDV IgG antibodies (Pro-Vitam)
- evaluation of antigenicity of the expressed HDV antigen with Elisa (Pro-Vitam)
- evaluation of antigenicity of two HCV antigens expressed by IBAR, using Elisa (Pro-Vitam)
- design of another Hepatitis Delta virus antigen, followed by expression and purification (Pro-Vitam)
A detailed progress report for phase 1 (in Romanian) can be downloaded from here.
The main activities undertaken in phase 2 of the project are the following:
- assembling the LFA strip for Hepatitis Delta antibody detection (IBAR)
- sensitivity and specificity tests on Elisa formulations using three Hepatitis Delta antigens (Pro-Vitam)
- sensitivity tests of the LFA strips (IBAR, Pro-Vitam)
- optimizing detection of HBsAg on LFA strips, combining it with HDV antibody detection (IBAR)
A detailed progress report for phase 2 (in Romanian) can be downloaded from here.
Results
Phase 1
- design and production of validated antigens for detection of anti-HDV antibodies
Phase 2
- assembled LFA strip for the detection of anti-HDV antibodies
- developed three Elisa assays, validated them in a clinical setting, and the best performing assay was found to have 100% sensitivity and 98.4% specificity for the detection of anti-HDV antibodies
Phase 3
- assembled lateral flow assays (rapid tests) for the combined detection of HBsAg and anti-HDV
- assembled lateral flow assays (rapid tests) for the combined detection of HBsAg and anti-HCV
- we created two fully functional prototypes of Elisa assays for anti-HDV and anti-HCV antibody detection (Technology Readiness Level 6). The assay will be licensed for production to the startup of Pro-Vitam Ltd, Proel Biotech.
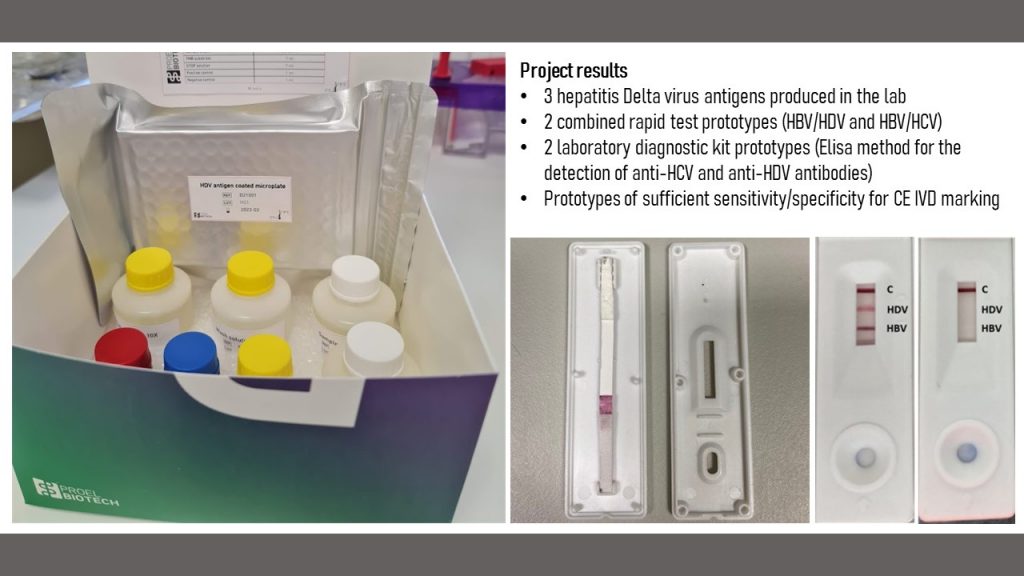
A detailed report of the project results (in Romanian, including all three phases) can be downloaded from here.
Events
Project results have been disseminated through an online workshop entitled “Rapid testing in the fight for viral hepatitis eradication” (in Romanian), on May 5th 2022.
Prothanor Biotech Ltd is a startup of the project team of IBAR and is undertaking the steps required for commercialization of the multiplex HBV/HDV/HCV rapid test. The company has been awarded a Leaders in Innovation Fellowship (LIF Global 2022), organized by the Royal Academy of Engineering in collaboration with UEFISCDI (https://www.raeng.org.uk/global/sustainable-development/leaders-innovation-fellowships/lifglobal).
Contact
Pro-Vitam Ltd: Dr Szilard Fejer, szilard.fejer@cantab.net, tel. +40728114907
IBAR: Dr Costin-Ioan Popescu, pop@biochim.ro, tel. +40212239069
2. Experimental demonstrator based on Raman spectroscopy for quantification of glycated proteins in diabetes screening (DiaScreen)
This project is implemented through a partnership with the Babes-Bolyai University (project coordinator) and Pro-Vitam Ltd. (project partener), and financed by CNCS-UEFISCDI (project number PN-III-P2-2.1-PED-2019-3268). The goal of the project is to develop a method based on Raman spectroscopy that is portable, easy to use in a clinical laboratory setting, and can reliably determine the quantity of glycated proteins (haemoglobin and albumin) in patient samples.
Group members
Babes-Bolyai University (proejct coordinator):
Pro-Vitam (project partner):
- Dr Szilard FEJER
- Dr Zsuzsanna JENEI
- Dr Kinga RAKOSI
- Monika KORODI (PhD student)
3. and 4. – research projects in partnership with the National Institute for Materials Physics
These projects have two main goals – nanosensor prototype development for detection of TSH and EGFR in patient samples, and studying the physico-chemical and antimicrobial properties of atorvastatin. The resulting publications can be found in the Publications section.